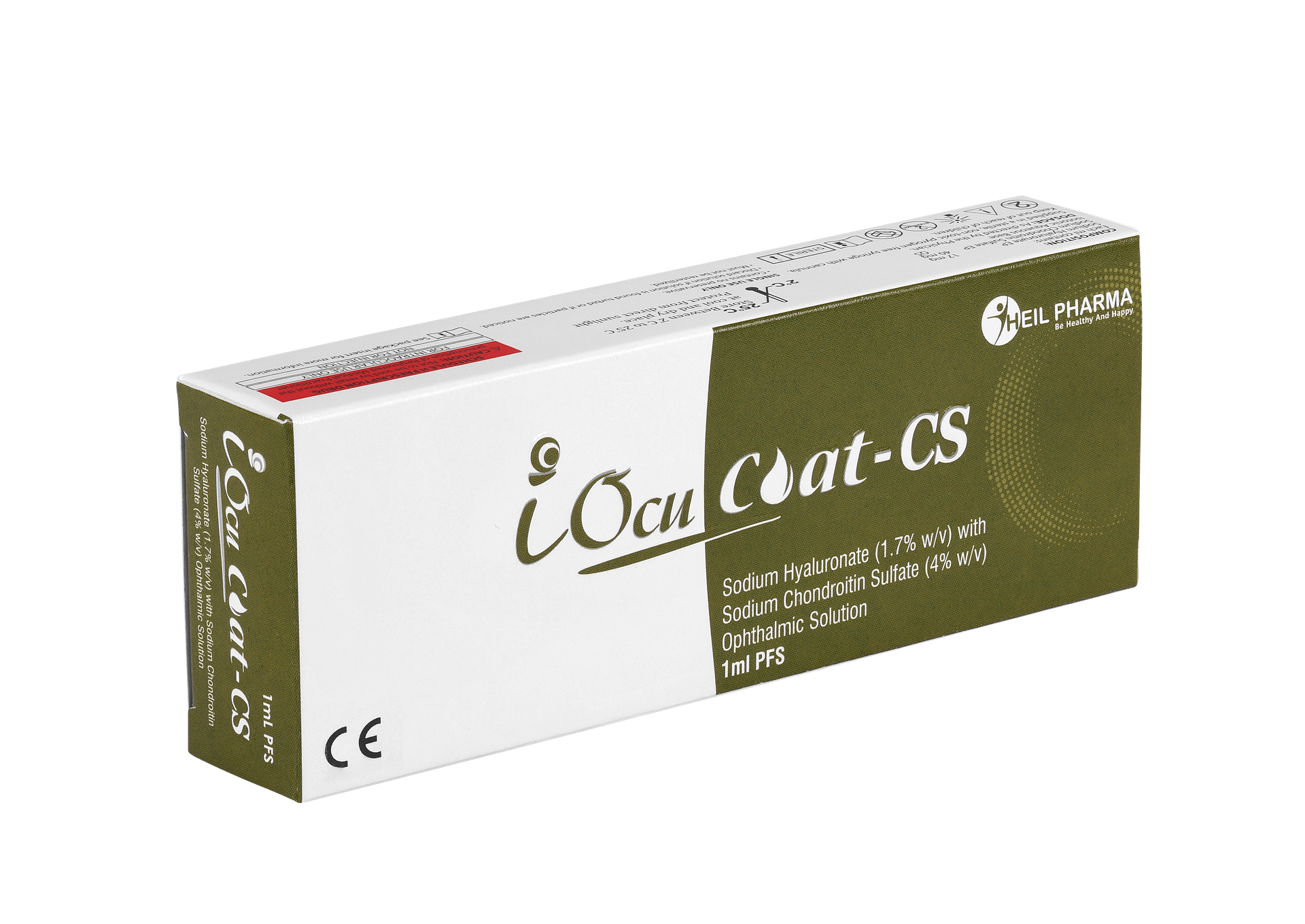
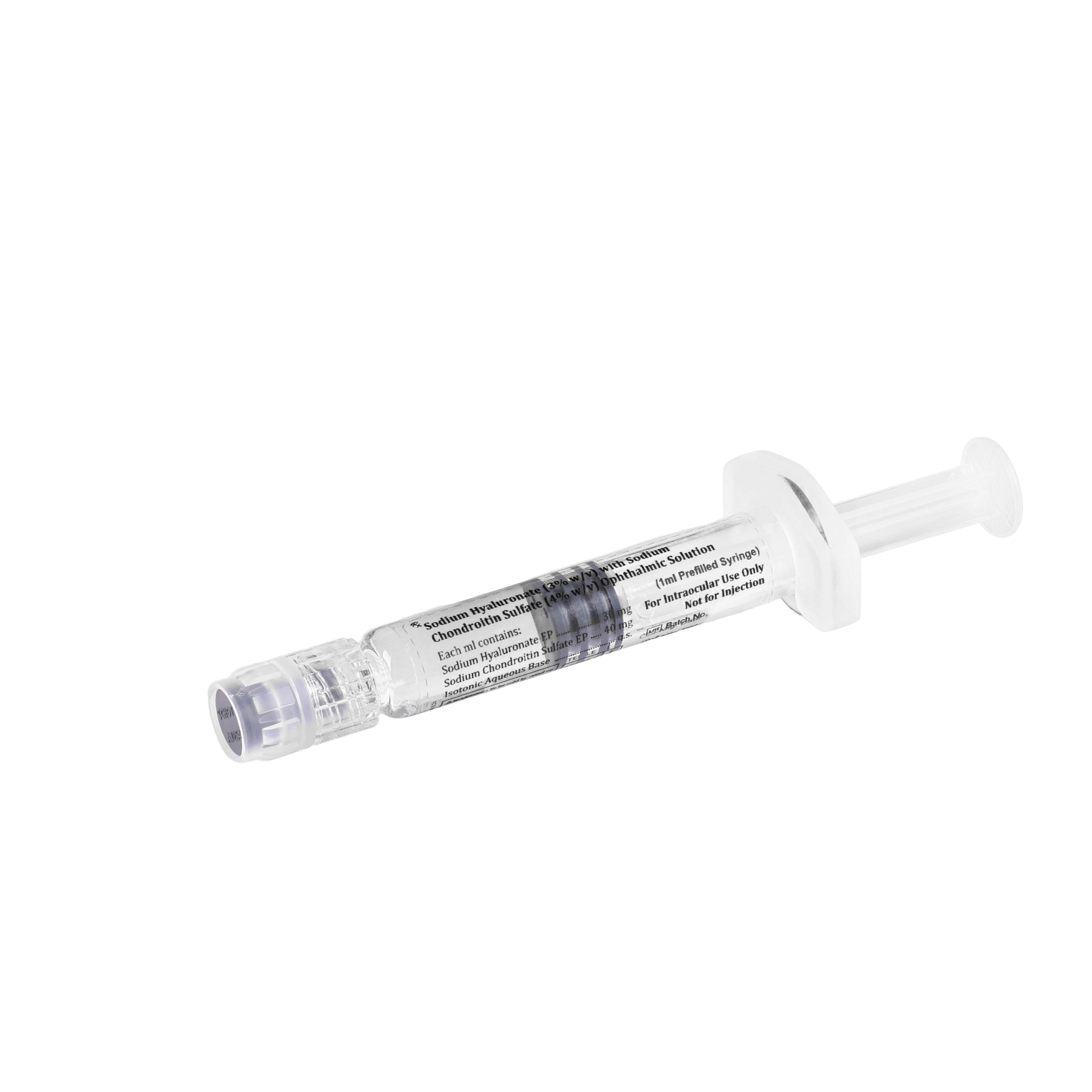

Sodium Hyaluronate (1.7% w/v) with Sodium Chondroitin Sulfate (4% w/v) Ophthalmic Solution – iOcuCoat-CS
iOcuCoat+ – Ophthalmic Viscosurgical Device has an intermediate cohesive/dispersive index (CDI). It is a sterile, non-pyrogenic, viscoelastic solution of a highly purified non-inflammatory medium molecular weight fraction of sodium hyaluronate and sodium chondroitin sulphate. iOcuCoat solution maintains a deep chamber during anterior segment surgeries, enhances visualization during the surgical procedure, and protects the corneal endothelium and other ocular tissues. The viscoelasticity of the solution maintains the normal position of the vitreous face, thus preventing formation of a postoperative flat chamber. It is designed to create and maintain space, to protect the corneal endothelium and other intraocular tissues and to manipulate tissues during surgery.
| Molecular Weight | High Molecular Weight |
|---|---|
| Viscosity at Rest | 200000 - 300000 mPas |
| pH | 6.8 – 7.6 |
| Osmolality | 325– 370 mOsm/Kg |
| Raw Material | Sodium Hyaluronate - Non-Animal Origin Sodium Chondroitin Sulfate - Non-Bovine Origin |
| Packing Material | Premium Quality of Pharmaceutical Grade |
| Available in | 1ml PFS |
